This article originally appeared on The Nexus blog. The Acid-Base Selection Tool can also be found alongside dozens of other tools for more sustainable chemistry on the GCS Tools & Metrics page.
Contributed by Charlotte Dalton, Scientific Group Leader at CatSci, Ltd.
The use of acids and bases is ubiquitous within the chemical and related industries, academic research, and everyday life. In fact, acid-base reactions are often the first chemical reactions taught and demonstrated in the science classroom, like discovering what happens when you mix baking soda and vinegar. Those who go on to study and work in the chemical sciences and related fields will likely find themselves having to select the optimum acid or base for their chemical reaction or process. What factors should you use to make this choice, and where can you find information to guide your decision? Usually, when we think about acids and bases, pKa (or pKb) is the instant focus. By tailoring the choice of acid or base strength to the job at hand, it’s possible to minimize side reactions and unwanted processes while the desired chemical reaction(s) take place smoothly with high yield. Other physical parameters of the acid or base may also be highly relevant depending on the process requirements, such as melting point, boiling point, or which functional groups are present.
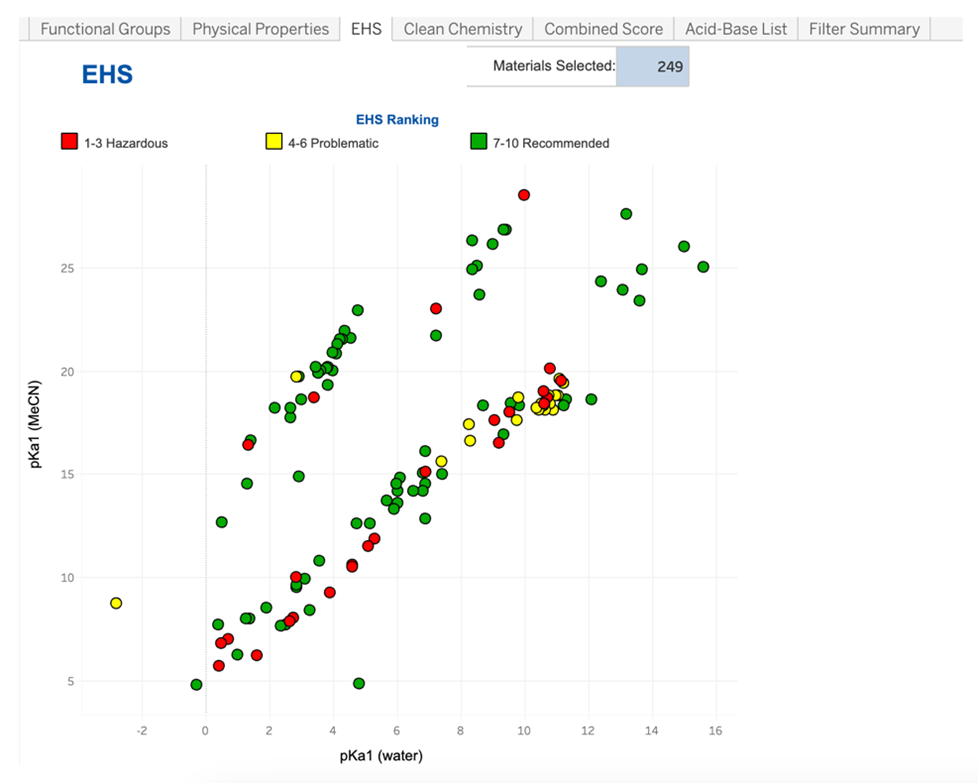
However, for the design of sustainable chemical processes, we should also consider the “greenness” of acids and bases. This means understanding which acids and bases are the least desirable from a sustainability perspective and seeking better alternatives. The new Acid-Base Selection Tool developed by the ACS Green Chemistry Institute Pharmaceutical Roundtable is now publicly available and aims to enable scientists across industry and academia to choose more sustainable acids and bases. Much like the Solvent Selection Tool, which will be familiar to many already, the Acid-Base Tool contains over 200 acids and bases, which the user can filter by parameters including pKa (in water or acetonitrile), functional groups, melting point, and boiling point. Crucially, the tool also contains scoring for the acids and bases on environment, health, and safety (EHS) and Clean Chemistry aspects. The scoring of the acids and bases was conducted as outlined in the article “Development of GSK's reagent guides – embedding sustainability into reagent selection.” The EHS score is based on hazard statements and occupational exposure limits for the materials, and the Clean Chemistry score is based on an assessment of ease of workup and atom efficiency, among other factors. A combined score is also presented for ease of comparison among the acids and bases.
We hope the tool will serve the scientific community by providing a comprehensive list of acids and bases scientists can use to identify suitable reagents for their specific purpose. In addition, it will encourage the selection of acids and bases with the most sustainable properties and help avoid the use of more problematic ones.
Questions and feedback on the Acid-Base Selection Tool should be directed to Charlotte Dalton or Vittoria Valentine, Industry Portfolio Administrative Assistant at the ACS GCI.
