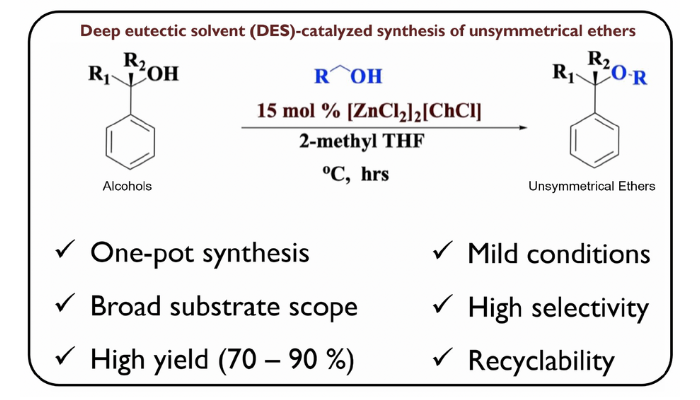
Unsymmetrical ethers are generally synthesized via the Williamson ether method, but the unwanted formation of symmetrical ethers plus the basic and harsh conditions of the route pose a synthetic challenge. Other methods employed in the synthesis of unsymmetrical ether require the use of toxic mineral acids, and requires high catalyst loading which limits their large-scale application. Dehydration of alcohols in the presence of base-metal catalyst has, however, recently offered the greenest approach to synthesize unsymmetrical ethers, leaving water as by-product. In this work, we describe our use of Lewis acidic zinc chloride in combination with choline chloride deep eutectic solvent (DESs) as catalyst for the dehydration of benzylic alcohol with other alcohols, to form unsymmetrical ethers. Ionic liquids employing base-metal catalysts, known as deep eutectic solvents (DESs), are low-price, recyclable, and offer high recovery in the synthesis of unsymmetrical ethers. Experimental data obtained from the catalytic cross-etherification of benzylic alcohols with various alcohols functional group, such as- nitro, allylic, methoxy, halide, thienyl and sterically hindered substrates gave a 75-95 % yield of unsymmetrical ethers subsequently. Also, the zinc-DES catalyst went through a 5-cylce re-run and gave about 80-90% yield of the unsymmetrical ether showing the high stability of the catalyst system. The use of this DES catalyst mixture provides a better approach from the green chemistry standpoint as environmentally benign, efficient, and recyclable catalyst in the synthesis of unsymmetrical ethers for broader industrial applications in surfactants, liquid fuels, polymers, and pharmaceuticals, to name a few.