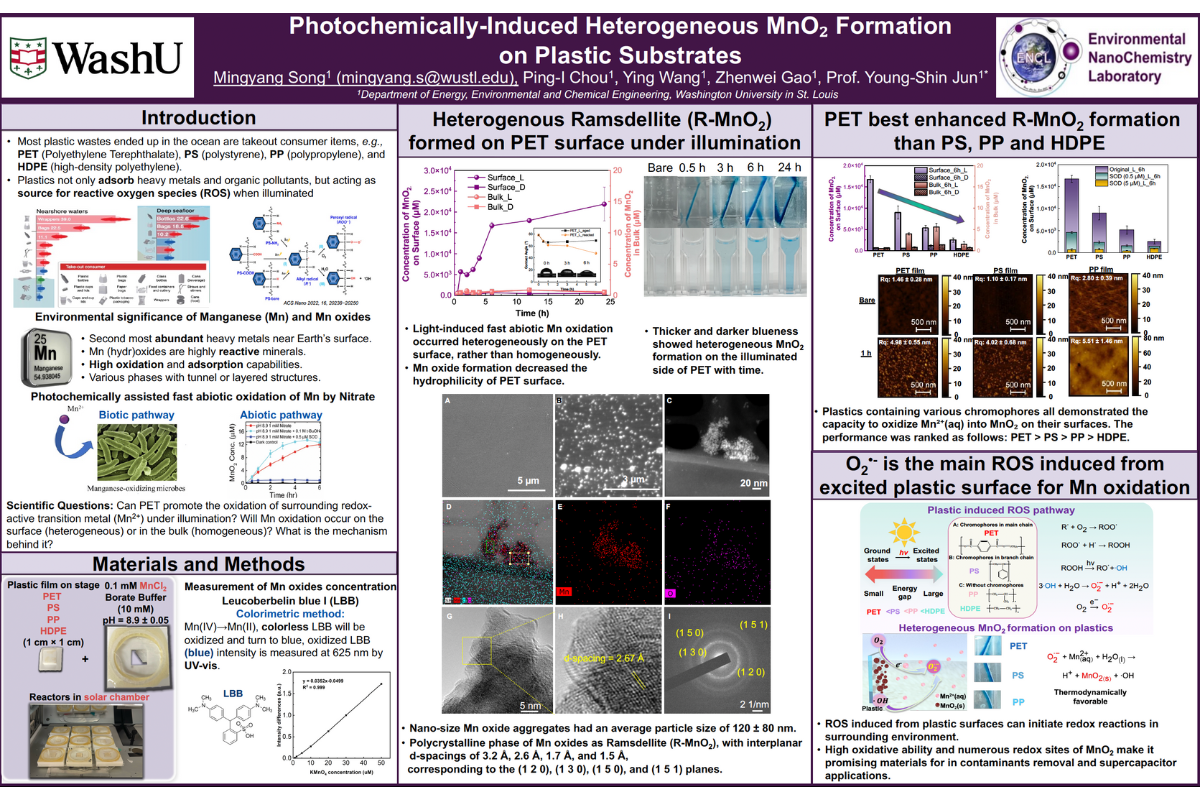
Natural sunlight governs the long-term fate of plastics in aquatic environments by altering their physicochemical properties, generating reactive oxygen species, and accelerating their breakdown into oxygenated products. However, interfacial photochemistry between plastic surfaces and naturally abundant redox-active metal—manganese (Mn) remains unexplored. Herein, we revealed the photo-oxidation mechanisms of Mn2+(aq) induced by surfaces of polyethylene terephthalate (PET) and other plastics (e.g., polystyrene (PS), polypropylene (PP) and high-density polyethylene (HDPE)). Within 6 hours, MnO2 vigorously formed onto the PET surface (1 cm × 1 cm) with concentration reaching 16,740 ± 810 μM under light exposure. Atomic force microscopy confirmed the fast MnO2 nucleation on PET within 1 h, increasing surface roughness to ~4.98 ± 0.55 nm. Both Raman spectroscopy and X-ray diffraction (XRD) determined that the transformed phase was Ramsdellite (R-MnO2). Among plastics with different chromophores, PET possessed the lowest energy gap chromophores, most effectively oxidized Mn2+(aq) by promoting ROS–superoxide radicals (O2•−) generation, the main contributor to Mn photo-oxidation. Furthermore, prolonged photoaging increased surface roughness and decreased hydrophobicity of plastic, further enhancing Mn oxidation. This study highlights how interfacial photochemistry of plastics influence spatial distribution of important redox-active transition metals in environments and mutually alter surface properties of plastics.