This article originally appeared on The Nexus blog.
By Cecilia Smith, Administrative Assistant, ACS Green Chemistry Institute

The European Chemicals Agency's proposed restriction aiming to ban PFAS stands to change how the pharmaceutical industry develops, produces, packages, and delivers medicines to patients around the world. The restriction, which aims to drastically reduce the use of persistent, harmful chemicals in industry, necessitates proactive efforts, efficient alternatives assessment, and collaboration within the pharmaceutical industry that has historically faced barriers to collaboration due to its many proprietary technologies. The ban also underscores the need for new research into greener synthetic pathways and hazard prediction to ensure immediate and long-term patient health while minimizing environmental impact.
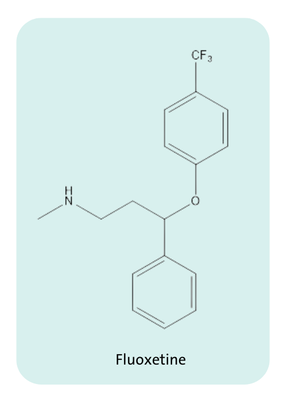
Per- and polyfluoroalkyl substances, commonly known as PFAS, are a group of over 10,000 synthetic chemicals with applications in industry and consumer products. The carbon-fluorine bond characterizing PFAS is one of the strongest bonds in organic chemistry. This stability has become critical for developing active pharmaceutical ingredients (APIs) that function properly and remain intact when moving through the harsh conditions of human metabolism. Fluoxetine, for example, an API commonly prescribed as an antidepressant, is one of 139 APIs considered a PFAS according to ECHA’s definition. The C-F bond stability in Fluoxetine is key to its effectiveness as an API as it prevents undesired metabolism of the drug in the body. However, the same C-F bond stability also prevents PFAS from biodegrading in the environment and has garnered the substances the nickname ‘forever chemicals.’
Unlike Fluoxetine, which has one fully fluorinated carbon atom, PFAS commonly used in commerce often have multiple fluorinated carbons contributing to their environmental persistence. The accumulation of these substances in the environment raises concerns because of the toxicity of certain PFAS—recent research suggests that environmental exposure to some PFAS can lead to health effects ranging from endocrine disruption to increased risk of cancer, and further present risks to aquatic life at environmentally realistic levels. Because of these harmful effects, regulatory agencies have begun to take action to mitigate human exposure to PFAS. In April of 2024, the EPA established unprecedented standards limiting the levels of six PFAS in drinking water. The European Chemicals Agency (ECHA) is also working to pass an even broader ban on the use and production of chemicals categorized as PFAS in Europe.
ECHA’s proposed restriction aims to ban the use of thousands of PFAS, with an exception for active pharmaceutical ingredients, and was initially intended to be implemented by 2028—five years after its proposal. As originally written, the proposed restriction would ban PFAS that are not APIs, including PFAS used as raw materials for making APIs. Though trade associations, lobbying groups, and representatives from pharmaceutical industries are advocating for exceptions, deadline extensions, and a more nuanced definition of PFAS to exclude certain use cases, it’s clear the restriction will bring major changes to the pharmaceutical supply chain.
For an API to go from a newly synthesized molecule to a pill in a consumer’s medicine cabinet, it relies on a number of PFAS containing materials, even if the API itself is not a PFAS. In drug research and development, PFAS can be found in coatings on laboratory instruments, key solvents and process materials, and pipework, seals, and gaskets in production equipment. But it doesn’t stop there. PFAS coatings are used in storage, transportation, pill blister packs, syringes, and injector pens that are handled by consumers. The reason PFAS are so broadly used in production and packaging is the same reason they make effective APIs: their bond stability and durability make them an ideal choice in an industry where it is crucial to minimize unintended side reactions and by-products for end-product purity.
John Wasylyk, Scientific Director at Bristol Myers Squibb, described the number of challenges that arise from something as small as an o-ring used in the API production process.
“When you’re running something in a flow process, there are o-rings and seals which need to be resistant to harsh chemicals and endure considerable torque from moving impellors, rotators, and mixers,” he said.
O-rings can’t be made of something that could readily degrade, since that would risk the introduction of foreign material into the API.
“If something was extracted from a non-PFAS-based seal, and that component dissolved and ended up in your drug, you have to embark on an impact investigation and go through a re-work and re-purification process, and in some cases you might have to dispose of the entire batch and begin a new process.”
Developing and testing an alternative that is as effective as the original PFAS seal therefore can become a time-consuming and costly problem, in addition to a challenge in maintaining API purity. Assessing all PFAS use cases across existing equipment and processes only compounds these challenges. Some companies, however, are pro-actively beginning this daunting task.
“With the OECD 2020 terminology paper, the definition of PFAS changed in EU. For many years ultra short chain PFAS and fluoropolymers were used without concern. So, there is a whole mapping process to figure out where and how PFAS is used in the pharmaceutical industry and how they can be substituted,” said Eva Vestergaard, Global Project Lead in Corporate Environmental Strategy at Novo Nordisk.
Cooperation with suppliers who provide PFAS coatings and production materials and who would be significantly affected by the proposed ban is also critical, according to Vestergaard.
In addition to better understanding their PFAS use, pharmaceutical companies are beginning to explore the possibilities and challenges of alternatives assessment.
“We will look for alternatives,” said Wasylyk, who noted that substitutions are also a concern for the industry as it navigates this process development and manufacturing. “You have to look at the whole toxicological picture and say, ‘What’s the next best modification to the drug or process we could substitute without causing deleterious effects?’”
When it comes to making the desired carbon-fluorine bond within an API, however, removing raw materials and intermediates with PFAS is even trickier.
“We will still need to make or utilize fluorinating reagents to put fluorine groups on drugs,” said Wasylyk. “We could use other halogens to avoid fluorine, but then you’re generating bromine, iodine, or chlorine and you’re going to have to dispose of another type of halogen.”
Despite the exemptions for APIs from the PFAS restriction—and the possibility of exemptions for PFAS reagents and intermediates—there is a desire among some researchers to find new synthetic pathways that avoid PFAS from the start.
“One of the purposes of green chemistry is to develop treatments that are good for patients but also protect the environment,” said Vestergaard. “Even though there is an exemption to PFAS APIs, I definitely think it’s worth doing research to look for alternatives.”
“Though we continue to learn lessons from the rational design of APIs to interface with Green Chemistry Principles 4 and 10 for reducing environmental risks of other anthropogenic contaminants, we need to advance the sustainability of health care by limiting the impacts of production, including PFAS co-contaminants, and minimizing the negative effects of APIs in ecosystems,” said Bryan Brooks, Distinguished Professor at Baylor University.
Though a major disruption to the industry, the restrictions can also be seen as an opportunity for improvement and innovation.
“We will find ways to make things better and reduce our reliance on fluorine containing compounds. It will take some time, but I think as chemists and engineers, we will rise to the occasion,” said Wasylyk.
The scope and breadth of the restriction proposal also introduces a somewhat unprecedented opportunity for collaboration among pharmaceutical companies. Since alternatives assessment and development is a time- and labor-intensive process, Wasylyk hopes that “companies are open about what they find works and what they find doesn’t work, in order to help drive the changes more quickly.”
Organizations like the ACS GCI Pharmaceutical Roundtable and IQ Consortium have fostered this collaboration between companies to promote human health and innovation, and according to Wasylyk, this degree of openness represents a significant shift in how the industry has traditionally operated.
As ECHA and the European pharmaceutical industry work through the nuances of the proposed PFAS restriction, it’s important to consider the ban’s implications for the United States and worldwide industry as well. Many U.S. pharmaceutical companies do research and development work in Europe, meaning ECHA’s restrictions will impact the U.S. supply chain.
“Historically, regulations that start in Europe often move to other parts of the world,” said Vestergaard. “Looking at what is coming in Europe is definitely worthwhile. You can’t just turn a blind eye to these initiatives.”
Though there are no broad regulations on PFAS used in industry in the United States, over 30 U.S. states have passed or are developing PFAS-related requirements in several industries. One of the main difficulties in creating these regulations will be ensuring they address the most concerning PFAS without taking away from efforts to improve sustainability in other capacities.
“There are highly hazardous PFAS and there are PFAS with no demonstrated hazards. Pushing to replace a PFAS simply because it is a PFAS risks diverting research and development time and effort that could be put to more productive use to improve sustainability in other aspects of the supply chain,” said Richard Engler, Director of Chemistry for Bergeson & Campbell, P.C., a law firm focusing on industrial chemical product approval and regulation.
As regulatory agencies and industries work together toward effective and meaningful PFAS policies, it’s clear that more mechanistic toxicology and environmental chemistry research will be needed to address the hazards of various PFAS analogs. On the pharmaceutical industry side, the ban also underscores the need to evaluate processes holistically. Doing so involves all stages of the supply chain and the end products themselves, making this process complex, time-intensive, and prone to regrettable substitutions. However, by working together toward a common goal of minimizing harm to human and environmental health, pharmaceutical companies, researchers, and policymakers have an opportunity to innovate for safer, more sustainable products that can be used worldwide.
