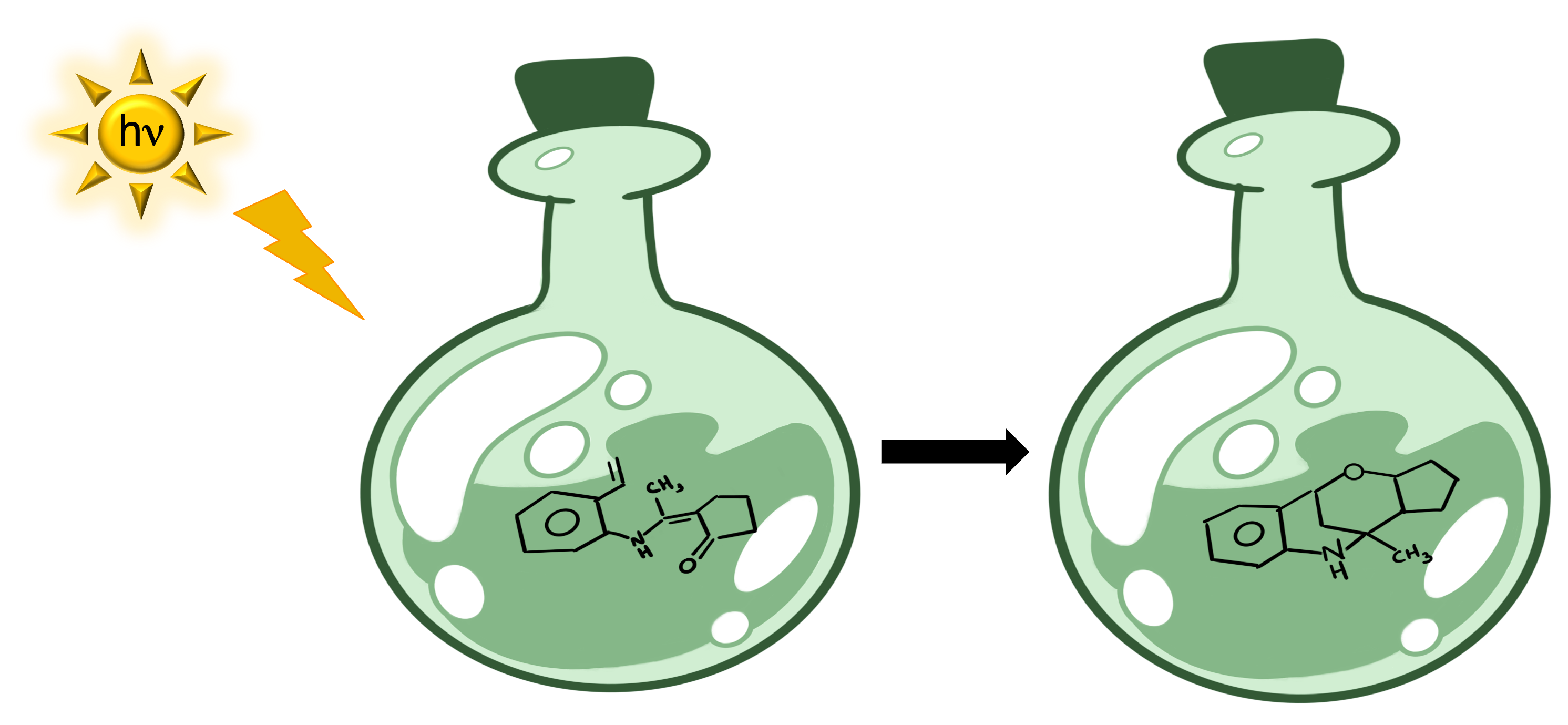
The use of light energy, which is considered a clean and traceless reagent, for the synthesis of complex heterocyclic molecules makes organic photochemistry one of the important green chemistry tools. Photochemistry holds an important role in accessing biologically relevant molecules containing complex skeletons that are difficult to access by conventional thermal methods. Skeletons that are difficult to obtain through ground state chemistry, can be constructed using light as a reagent, producing high energy excited states required for the molecules to undergo required transformation. Synthesis of several value-added chemicals and natural products requires photochemical reactions as a key step such as photocyclization, paterno-buchii reaction, photoene reaction, de mayo reaction.
De Mayo reaction is one of the well-known photo transformations reported by Paul Jose De mayo in 1962, where a [2+2] photoreaction between alkene and 1,3-dicarbonyl compound leads to the formation of cyclobutene, which undergoes retro-aldol reaction to form 1,5-dicarbonyl compounds. A diverse set of natural products are synthesized through the traditional [2+2] photo reactivity of diketones and alkenes such as (±)-ingenol, the alkaloid mesembrine. Upon changing the nature of the excited state, a new photo reactivity is observed leading to the formation of a dihydropyran photoproduct which resembles the core of anticancer drug candidate Marmycin A. Recently, Sivagruru and coworkers reported this new excited state reactivity where they have successfully accessed the core of an anticancer drug candidate in a single step using light as a reagent. The use of planar reactants for the synthesis of complex heterocyclic anticancer drug candidates gained importance as this bypasses harsh environmental conditions used for conventional thermal synthesis.
On excitation of the in situ formed enaminone intermediate, one of the major pathways of deactivation of the excited energy is isomerization. We hypothesized that on constricting this isomerization by employing cyclic diketones and by varying the size of the cyclic system, which can add in regioselectivity in the system. Cyclic b-diketones were employed with varying ring size and synthesis of the core of Marmycin A from cyclic b-diketones and amino alkenes using light energy was evaluated, where chemo selectivity of the formed photoproduct is dictated by the enaminone intermediate generated in situ. The intermediate formed is highly depended on the nature/size of cyclic 1,3-diketones employed and can be rationalized based on the Dieckmann-Kon rule. As we change the ring size of cyclic 1,3-diketones from five to six, a clear change in the chemo selectivity of the formed enaminone intermediate is observed, which reflected the regioselectivity in the formed photoproduct. Exploiting the Dieckmann-Kon rule, in which six membered enaminone ring systems generally prefer endocyclic double bonds as compared to five membered enaminone ring systems, which favor exocyclic double bonds, enaminones were synthesized and were subjected to light irradiation. Formation of a photoproduct featuring a bridgehead methyl substituent as a major isomer is observed in the case of five membered enaminones and a vinylic methyl substituent as a minor isomer. On the contrary irradiation of the six membered enaminones, resulted in the reversal in photoproduct formation, where dihydropyran photoproduct with vinylic methyl substituent is observed as the major isomer and a bridgehead methyl substituent as the minor isomer with moderate to good yields. The effect of solvent and temperature on the reactivity and preliminary photophysical studies revealed the possibility of the ESIPT mechanism invoked earlier. Further photophysical studies are under investigation to gain more insights to the mechanism of the reaction.