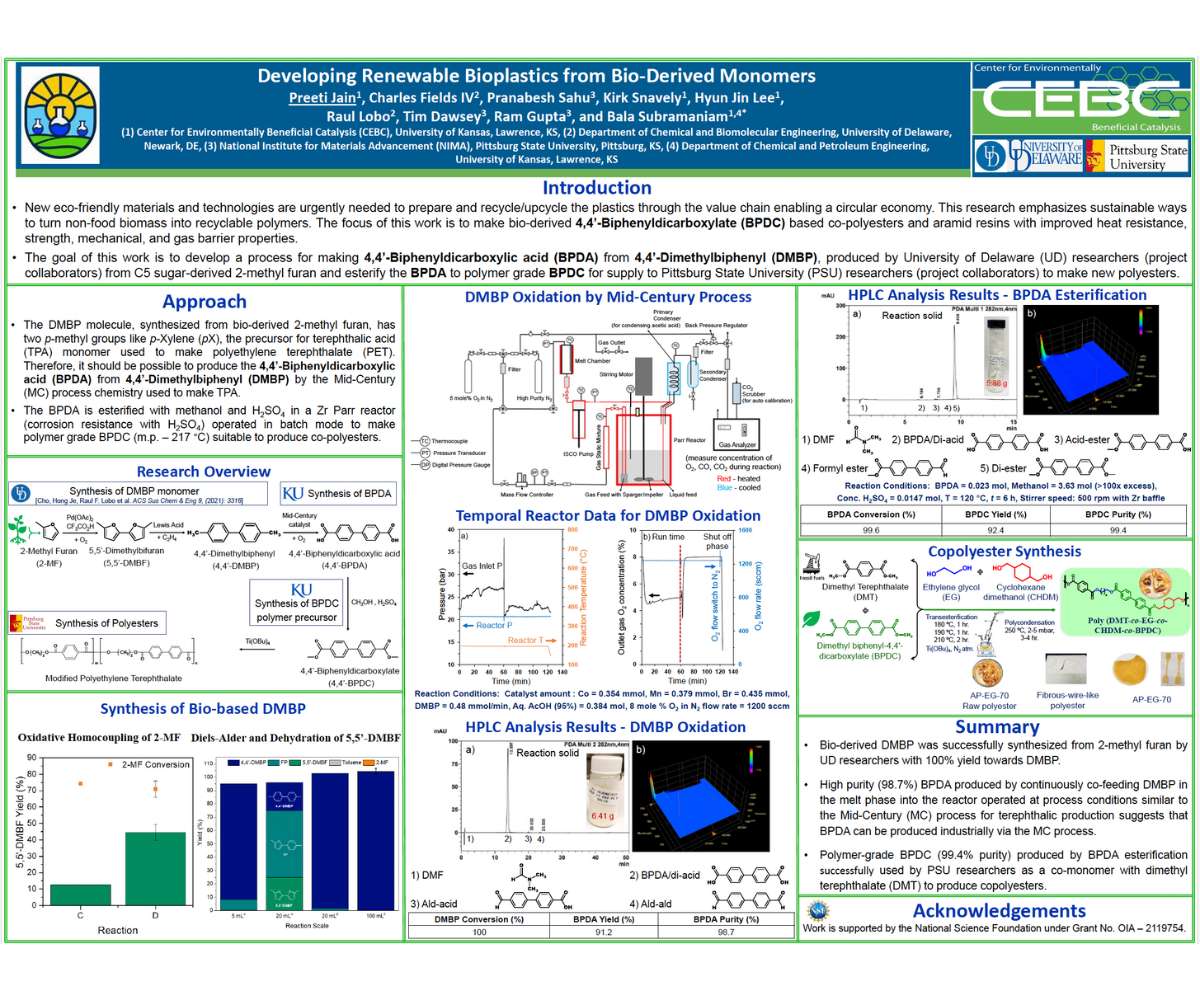
Enhancing the efficiency of material processing, utilization, and recycling is pivotal for advancing sustainability in modern society. Therefore, new eco-friendly materials and technologies are urgently needed to prepare and recycle/upcycle plastics through the value chain enabling a circular and sustainable economy. The objective of this study is to create sustainable methods for converting non-food biomass into recyclable polymers. One key compound, 4,4'-biphenyldicarboxylic acid (BPDA), is used as a polyethylene terephthalate (PET) additive in copolymer production or a feedstock to produce polyesters with improved properties. BPDA was synthesized through the oxidation of 4,4'-dimethylbiphenyl (DMBP), a compound that can be efficiently derived from biomass-sourced 2-methylfuran. An esterification process was used to obtain polymer grade 4,4'-biphenyl dicarboxylate (BPDC/diester) from BPDA. Further, the diester was blended with dimethyl terephthalate (DMT), to produce copolymers with either similar or superior properties to commercial PET resins. Palladium acetate was used as a catalyst in the oxidative coupling of 2-methylfuran with oxygen, and La-triflates were employed to catalyze the tandem Diels-Alder-Dehydration of 5,5’-dimethylbifuran and ethylene to form DMBP, yielding 83% DMBP in 18 hours. The extension of the Mid-Century (MC) process (used to produce terephthalic acid monomer from p-Xylene) to selectively oxidize DMBP to the BPDA monomer is non-trivial as DMBP is a solid at room temperature. Hence, DMBP was continuously fed into a stirred reactor in the molten state and oxidized at Mid-Century (MC) process conditions using Co/Mn/Br salts dissolved in acetic acid at 20 bar and 195 °C. BPDA was obtained in solid form at 91.2% yield and 98.7% purity and with complete DMBP conversion and ~92% carbon balance. Our findings thus indicate that the industrial production of BPDA can be achieved via the MC process. Subsequently, BPDA was esterified with methanol to yield 85% 4,4'-biphenyldicarboxylate (BPDC) with 99.7% purity. Polymerization of BPDC is then performed by combining 20-30% BPDC as a co-monomer with dimethyl terephthalate (DMT) in ethylene glycol, catalyzed by Ti(OBu)4. This results in a blended polyester suitable for applications in films and fibers, demonstrating the potential for sustainable polymer production from biomass-derived feedstocks.