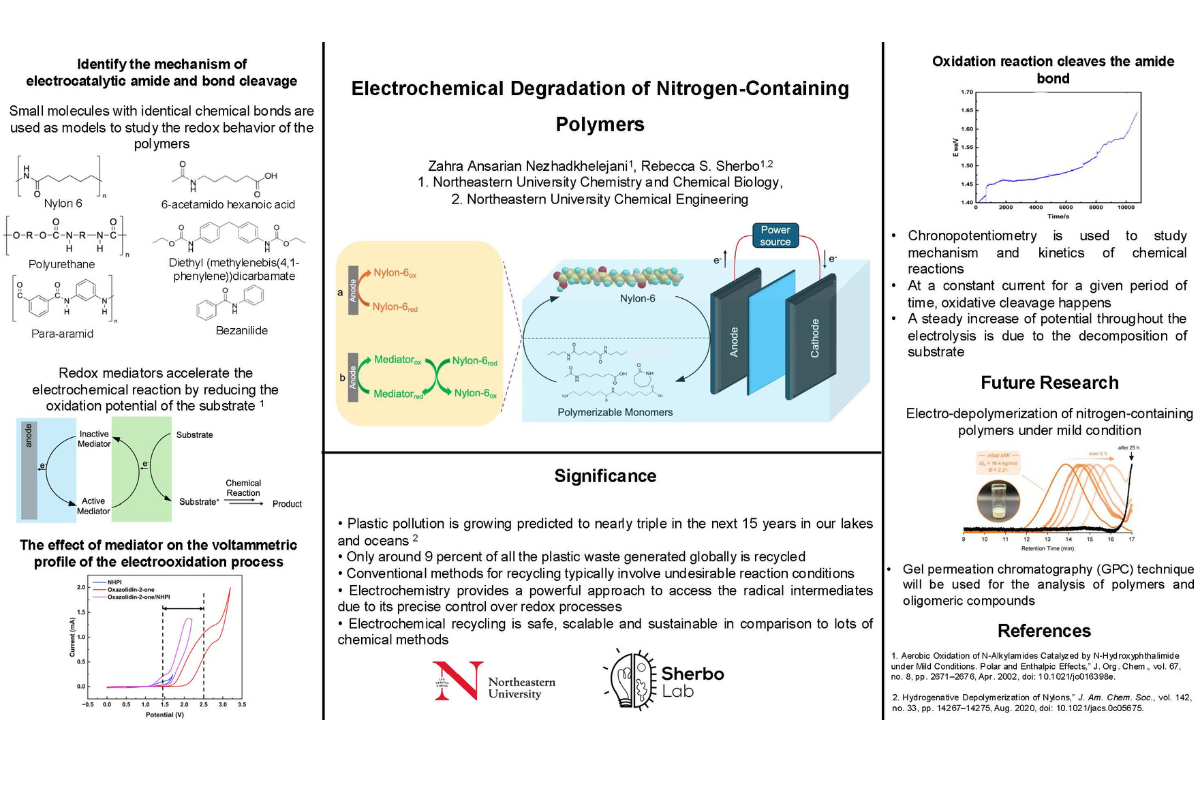
The natural environment is facing several contaminants including hazardous metals, dyes, medicines, and plastics. In particular, plastics, one of the most sought-after synthetic materials, are widely used in a variety of applications, including electronics, building, and packaging, due to their ease of manufacture and low weight. One novel recycling method that has been introduced as a mild and sustainable technology for processing waste plastic is electrochemistry, particularly when driven by renewable energy sources. In this work, we present the first electrochemical depolymerization of nitrogen-containing polymers (para-aramid, polyurethane, and nylon) into reusable monomers under mild conditions. We evaluate this electrochemical decomposition through solvent screening, electrolysis using the redox mediators, model substrate studies, and control experiments. Before investigating the deconstruction of the actual polymer, we study the electrochemical oxidation of the small molecule model to get insight into the degradation mechanism of the polymer. Also, to understand the reaction mechanism taking place during the anodic oxidation, identification analysis such as GCMS, and 1HNMR is employed.