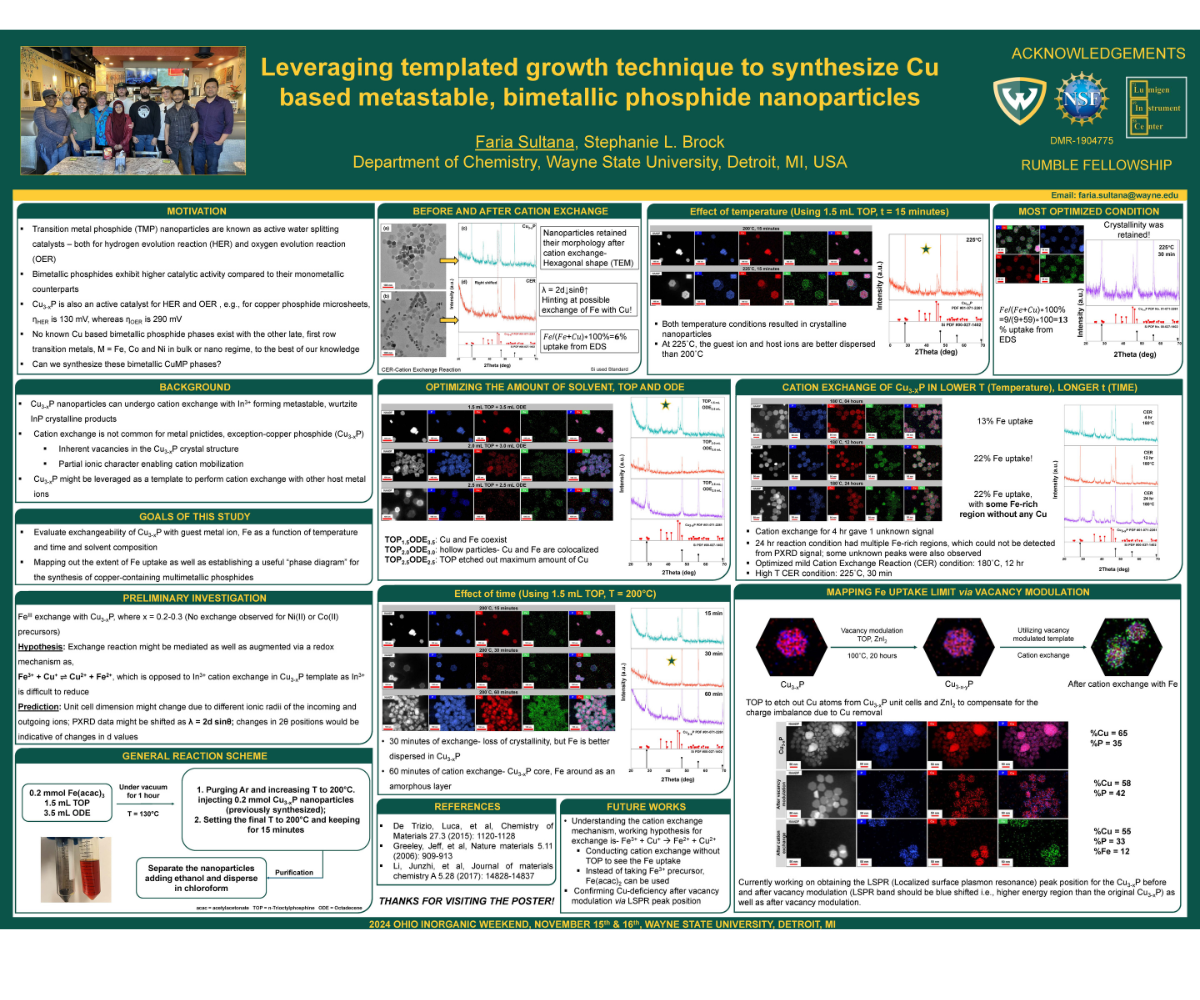
The existing global energy crisis demands potential materials for applications relating to renewable energy production, for instance, hydrogen fuel generation via water splitting. Transition metal phosphide (TMP) nanoparticles e.g., copper phosphide (Cu3-xP), nickel phosphide (Ni2P), etc. are well known water splitting catalysts. Our prior experiences with TMPs confirm the superior activity of bimetallic phosphides over their monometallic counterparts. To date, there is no well-defined, bimetallic copper containing phosphides in bulk phase with the other late, first row transition metals-Fe, Co, Ni. Therefore, synthesizing these metastable, bimetallic phosphides on the nanoscale may open the door for the fundamental understanding of their activity, including but not limited to, as potential catalyst for water splitting.
Inspired from recent works demonstrating the formation of metastable, wurtzite indium phosphide (InP) nanocrystals from Cu3-xP (having inherent vacancy and partial ionic character) via cation exchange, we began our effort to synthesize new bimetallic TMP by partial exchange with Fe3+. To demonstrate the feasibility of Fe uptake in Cu3-xP, different parameters (time, reaction temperature, solvent concentration) were varied. We observed 9% Fe incorporation with the most optimized reaction condition (225˚C, 30 minutes); we also investigated the cation exchange reaction under milder reaction condition (180˚C, 12 hr), that surprisingly ended up having more (15%) Fe uptake!
Our current effort is to map out the extent of Fe uptake in Cu3-xP template maintaining its crystallinity. To do so, we are using the tool “Vacancy Modulation”, where we are extracting more Cu atoms from the already Cu-deficient Cu3-xP, and those newly formed vacancies may provide more sites for enhanced guest ion uptake in the copper phosphide template.