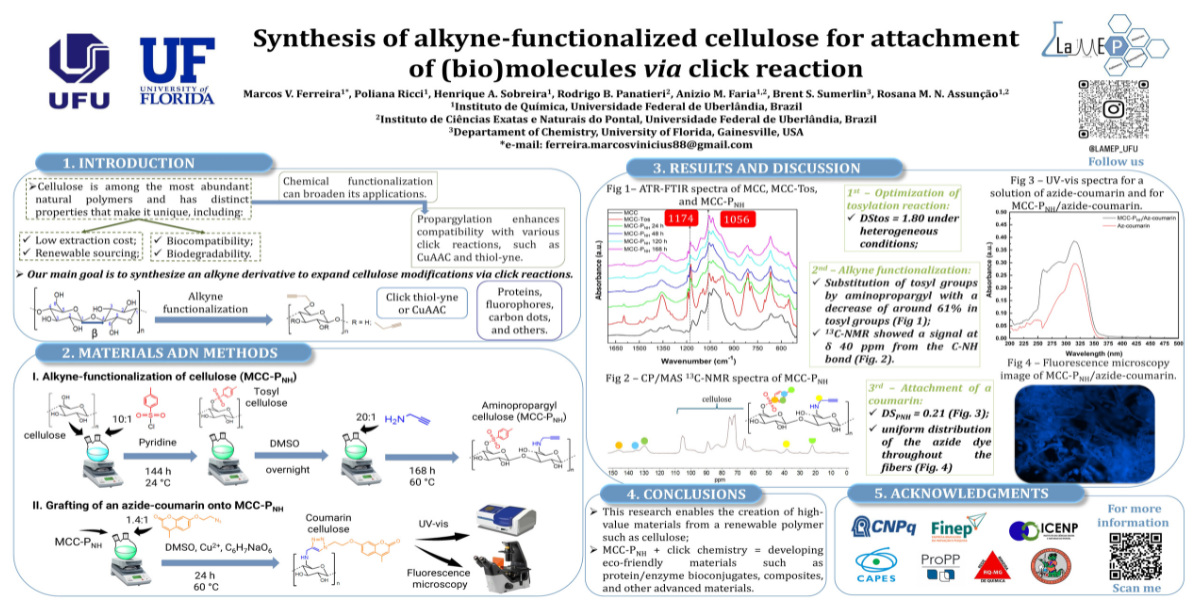
The development of sustainable and environmentally friendly materials is a key component of the green chemistry concept. In this context, cellulose tosylate (MCC-Tos) serves as a versatile precursor for the functionalization of cellulose. By substituting tosyl groups with alkyne groups, the potential of cellulose is enhanced, making it compatible with click chemistry reactions such as thiol-yne and copper-catalyzed alkyne-azide cycloaddition (CuAAC), which promotes greener processes.
This study optimized the heterogeneous synthesis of MCC-Tos using a Doehlert matrix statistical design, allowing for a systematic evaluation of reaction conditions to minimize waste and energy consumption. Under optimal conditions—specifically, a reaction time of 144 hours, a molar ratio of 10:1, and a temperature of 30 °C—a high degree of substitution (DStos = 1.80) was achieved.
Subsequent functionalization with propargylamine resulted in the creation of aminopropargyl cellulose (MCC-PNH), which reduced the degree of substitution by 65%. The successful attachment of alkyne groups was confirmed through 13C CP/MAS NMR. This was further validated by performing a copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) with an azide-functionalized coumarin. Fluorescence microscopy and UV spectroscopy estimated a degree of substitution of 0.21.
This work demonstrates a green and efficient pathway for advancing the development of sustainable materials, aimed at creating eco-friendly bioconjugates, composites, and innovative materials utilizing thiol-yne and CuAAC reactions with cellulose as a platform.